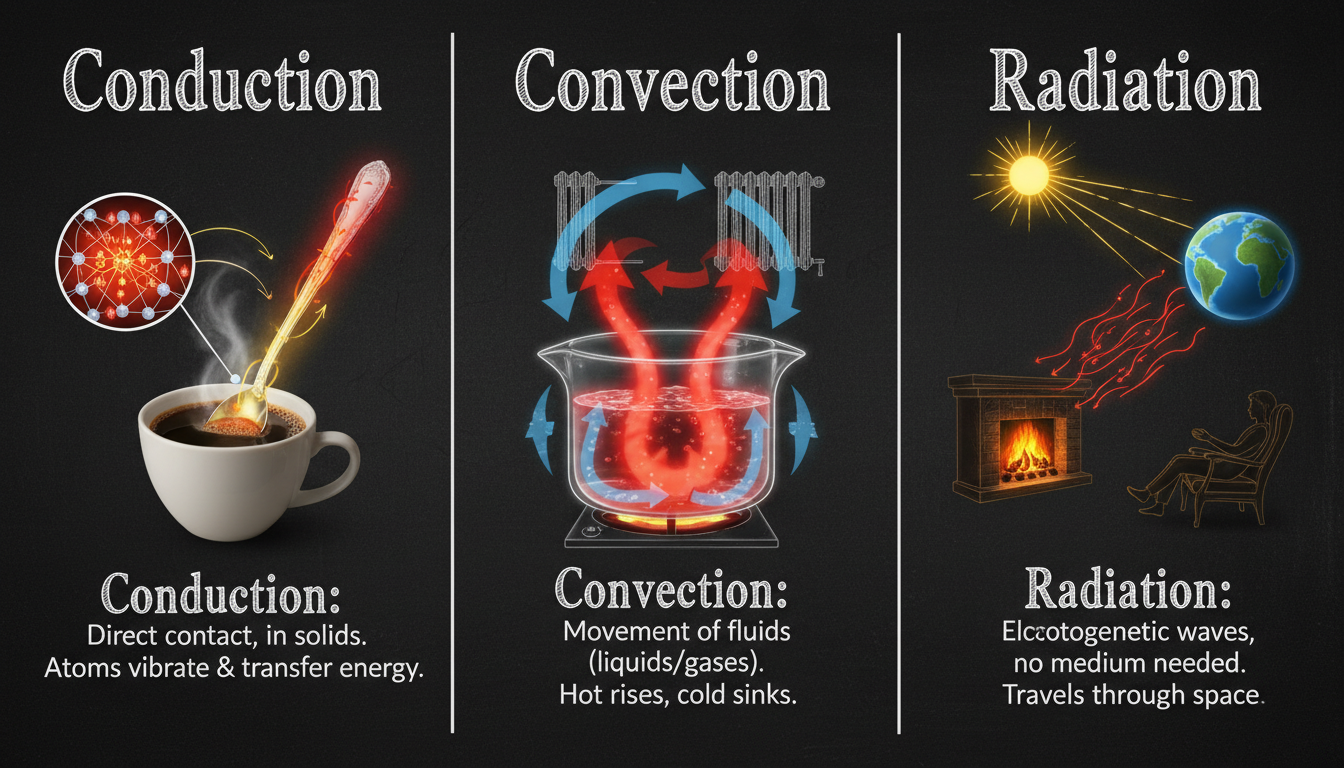
Thermal energy often gets tossed around in physics classes or documentaries, yet it’s surprisingly elusive for many. Sure, it’s energy related to heat—but what exactly does that mean? Let’s untangle this in a way that feels less like a lecture and more like a conversation over a steaming cup of coffee. You’ll walk away with practical insights, a dash of curiosity, and maybe even a tiny “aha!” moment.
What Thermal Energy Really Means
Defining Thermal Energy in Everyday Language
At its core, thermal energy refers to the total kinetic energy of particles within a substance. Basically, speed of tiny particles—atoms and molecules—add up to create what we sense as temperature. But it’s not just about motion; thermal energy reflects how much internal energy is buzzing around in a given system.
Different from Heat and Temperature
Here’s where things get sticky (and humanly confusing sometimes). Temperature is a measure—a reading on your thermometer. Heat, on the other hand, is the transfer of thermal energy, flowing from warmer to cooler objects. So when you hold a cup of tea, warmth transfers to your palm—that’s heat in action. Thermal energy is the broader concept behind both.
How Thermal Energy Shows Up Around You
Real-World Examples
- A simmering pot of soup: the rising steam and warmth represents thermal energy in motion.
- Holding an ice cube—cold energy leaving and warming your fingers.
- Combustion engines—thermal energy from burning fuel powers your car’s pistons.
These examples highlight how thermal energy underpins countless everyday experiences, from cooking to commuting.
Everyday Impact and Benefits
Beyond obvious heat, thermal energy powers HVAC systems, allows for solar thermal panels, and even underpins geothermal plants. It’s often overlooked—but quietly drives efficiency and sustainability in modern infrastructure.
The Science Behind Thermal Behavior
Microscale Motion in Materials
Digging deeper, materials with lighter molecules, like gases, tend to vibrate more vigorously and store thermal energy differently than heavier solids. That’s why gases expand and rise when heated, while denser materials may conduct heat more slowly.
Phase Changes with Thermal Energy
When ice melts or water boils, you see direct conversion of thermal energy. Those phase changes—solid to liquid, liquid to gas—absorb or release energy without changing temperature, which can feel counterintuitive until you see it in action.
“Thermal energy is simply the measure of particle motion—how much jiggling is happening inside.”
This sums up the essence, in a nutshell. Just imagine trillions of particles shaking and bumping around, and that’s the heat buzzing through.
Why Thermal Energy Matters Today
Real-World Applications for a Sustainable Future
- Geothermal systems leverage thermal energy deep in the earth, offering steady, low-carbon power.
- Solar thermal panels capture and convert sunshine into heat for water and air systems.
- Advanced insulation materials slow heat loss, boosting energy efficiency in buildings.
Contemporary Relevance
With global concerns over energy waste and carbon footprints, thermal energy earns respect in clean-tech strategies. It’s not flashy like AI or rockets, but it’s quietly essential, with growth that’s gradually picking up steam.
Navigating Common Misconceptions
It’s Not Just About Hot and Cold
Thermal energy isn’t a thing you can hold—it’s purely the movement inside. People sometimes say “cold is a lack of heat,” but that phrasing can create confusion. In reality, cold is absence or reduction of thermal energy.
Quantity vs. Intensity
You might feel surprised when a large body of cold water cools slowly compared to a drop of hot oil burning you fast. That’s the difference between total energy content (quantity) and temperature (intensity).
Visualizing Thermal Energy in Practice
Heating Water—A Case Study
Boiling a kettle illustrates many thermal energy principles: heat transfer from burner to metal to water; phase change; energy needed to overcome molecular bonds. By observing steam, you’re watching thermal energy make molecules dance their escape routine.
Building Design and Insulation
High-performance insulation resists thermal energy flow. Walls, windows, and roofs with better insulating materials slow down heat exchange, trimming heating or cooling demand—an everyday example of thoughtful engineering.
Wrapping Up with Insight
To sum up: thermal energy is motion. It propels storms and warms our coffee cups. It keeps buildings cozy—or sweating—depending on design. It transitions states of matter, drives industrial systems, and even anchors renewable technologies. Grasping its essence shifts how we interpret everyday phenomena and imagine future innovations.
FAQs
What is thermal energy in the most basic terms?
Thermal energy refers to the total internal kinetic energy of particles in a substance, reflecting how much motion exists at the microscopic level.
How does thermal energy differ from heat?
Heat is the process of transferring thermal energy between objects, while thermal energy is the stored energy within an object.
Why doesn’t temperature always change during a phase change?
During phase changes, thermal energy goes into breaking or forming bonds between particles rather than raising temperature.
Can thermal energy be used directly as electricity?
Not exactly—thermal energy often needs conversion, through methods like thermoelectrics or steam turbines, to generate electricity.
How do insulation materials relate to thermal energy?
They slow or minimize the transfer of thermal energy, helping maintain desired temperature levels inside buildings or containers.
Is thermal energy renewable?
In many applications, like geothermal or solar thermal systems, thermal energy harnesses renewable heat sources, contributing to sustainable energy strategies.


 February 7, 2026
February 7, 2026  5 Min
5 Min  No Comment
No Comment