Digging into the cellular respiration equation feels almost foundational—like a secret handshake that every biology student eventually learns. But beyond memorization, understanding the equation—what it reveals and what it hides—can reshape how you perceive energy flows inside every living cell. Mistakes happen, words get tangled (“is it 38 ATP or 30?”), and that’s okay—curiosity drives deeper clarity. Let’s unpack the formula, the actors (reactants and products), and the broader dance of energy that makes life tick.
The Simplified Equation: What Cells Do, in a Nutshell
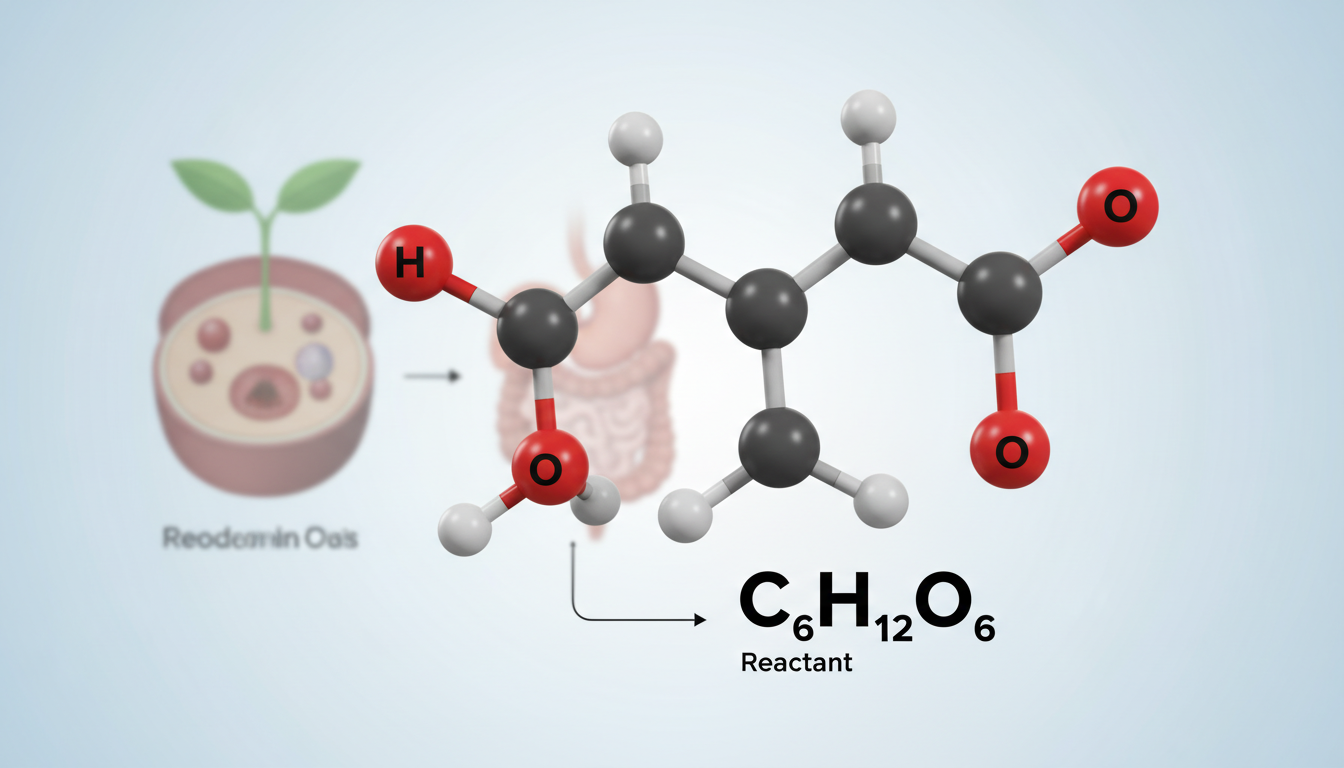
At its core, the equation reads:
text
C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O + ATP (energy)
Here’s the gist:
- Glucose (C₆H₁₂O₆) and oxygen (O₂) are the key inputs—call them the fuel and ignition.
- Carbon dioxide (CO₂) and water (H₂O) come out as waste byproducts.
- And yes, ATP—adenosine triphosphate—is the golden ticket: usable energy.
This version doesn’t detail every intermediate step, but it nails the big picture—what goes in, what comes out, and what’s gained.
Why This Equation Works: Redox Reactions Unveiled
Beyond a formula, it’s an oxidation-reduction (redox) reaction. Glucose is oxidized (loses electrons), while oxygen is reduced (gains electrons), making this the engine of ATP production. It may sound mechanical, but these electron transfers offer a neat peek into how chemical potential transforms into the delicate energy currency our cells run on.
Breaking It Down by Stage: Glycolysis, Krebs, and Beyond
Let’s go a bit deeper—because that simplified equation is really a summary of three major acts:
Glycolysis (in the cytoplasm)
- Glucose splits into two pyruvate molecules.
- Yields a small heap of ATP and NADH.
Citric Acid (Krebs) Cycle (in mitochondria)
- Pyruvate morphs into acetyl-CoA and enters the cycle.
- Spins out NADH, FADH₂, a little ATP (or GTP), plus CO₂.
Electron Transport Chain & Oxidative Phosphorylation (inner mitochondrial membrane)
- NADH and FADH₂ hand off electrons; oxygen becomes water.
- Proton gradient forms; ATP synthase turns ADP into ATP.
What’s fascinating here is that the “ATP” in the summary equation is an aggregate of complex processes—so when you see ATP in the output, realize it’s a product of a multi-step production line, not just one reaction.
How Much ATP, Exactly?
Textbooks often throw around “38 ATP per glucose,” but that’s more of a theoretical peak. Real-world efficiency drops because of leaky membranes and energy costs like transportation of molecules across mitochondria. Most estimates now hang around 29–30 ATP per glucose.
So that bold “+ ATP” in the equation? It’s shorthand that hides nuance—and a few losses—so it’s best taken as an overview rather than a hard count.
Why the Equation Still Matters in Everyday Biology
Even if you don’t need to know every NADH or proton gradient detail, the equation is more than biochemical shorthand—it’s a storytelling tool about how cells harvest energy, how oxygen is vital, and how we breathe out CO₂ in exchange for living.
Here’s a mini real-world reflection: imagine aerobic exercise—your muscle cells are ramping up that formula like a factory put into overdrive. More glucose and oxygen are pulled in, more CO₂ is expelled, and ATP churns out to fuel every muscle contraction you make.
“Understanding the cellular respiration equation is like mastering the narrative arc of how cells extract, convert, and utilize energy—it’s biology’s energy economics.”
—Acellular researcher on the importance of biochemical storytelling.
FAQs
What are the main reactants and products?
The primary reactants are glucose (C₆H₁₂O₆) and oxygen (O₂). The main products are carbon dioxide (CO₂), water (H₂O), and energy in the form of ATP.
Does the equation tell us the ATP yield?
Not exactly. It summarizes key reactants and products, but the actual ATP yield (usually ~29–30 ATP per glucose) depends on efficiency and losses in real cells.
Why is oxygen important in the overall equation?
Oxygen acts as the final electron acceptor in the electron transport chain—without it, the chain stalls and ATP production drops sharply.
Is anaerobic respiration the same equation?
No. Anaerobic respiration or fermentation doesn’t use oxygen and produces much less ATP (around 2 per glucose) alongside different byproducts like lactic acid or ethanol.
Conclusion: Why That Equation Still Matters
The cellular respiration equation—C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O + ATP—serves as a compact yet powerful statement of how life converts fuel to energy. It’s a scaffold that connects molecules, cellular structures, and physiological questions: How do muscles get powered? Why is oxygen so vital? Why does breathing matter?
Despite its simplification, it anchors our understanding and invites us to explore deeper layers—redox math, mitochondria dynamics, ATP efficiency. So next time you encounter that equation, think of it less as a static line and more as a doorway into the elaborate choreography that keeps every living cell running.
FAQs
What is the balanced chemical equation for cellular respiration?
It is ( C_6H_{12}O_6 + 6 O_2 \to 6 CO_2 + 6 H_2O + ATP ), summing up how glucose and oxygen transform into carbon dioxide, water, and usable energy.
How does glycolysis differ from oxidative phosphorylation?
Glycolysis happens in the cell’s cytoplasm and gives a small amount of ATP and NADH. Oxidative phosphorylation happens in mitochondria, generating the bulk of ATP via the electron transport chain.
Why doesn’t the equation specify how many ATP molecules are made?
Because actual ATP yield varies by cell type and efficiency. The equation indicates energy output, but the real-world figure (~29–30 ATP per glucose) reflects complex inefficiencies.
How do anaerobic pathways compare?
Anaerobic pathways bypass oxygen and yield only about 2 ATP per glucose. They produce unique byproducts depending on the organism, like lactic acid in humans or ethanol in yeast.


 February 6, 2026
February 6, 2026  5 Min
5 Min  No Comment
No Comment