You’ve probably encountered the term “ion” somewhere—maybe in a chemistry class, in health discussions, or while fiddling with electronics—and wondered: what exactly is an ion? Let’s unravel that together. There’s a neat precision to the concept, but real-world usage often comes with quirks, the odd anecdote, and sometimes just a tiny mistake from the human side of things. You might think of ions as just charged bits of matter—but they’re far more fascinating, weaving through fields from biology to space travel. Let’s dive in.
What Is an Ion? Simple Definition and Scientific Roots
At the most basic level, an ion is (drumroll, slightly imperfectly) an atom or molecule that carries a net electric charge—because it’s lost or gained electrons. When electrons don’t match protons, poof—you have an ion.
There are two main flavors:
– Cations—positively charged ions, missing one or more electrons (e.g., , a sodium ion)
– Anions—negatively charged ions, having gained electrons (e.g., , a chloride ion)
Beyond the definitions, there’s some history. Michael Faraday coined the terms cation (“moving down”) and anion (“moving up”) in the 1830s, inspired by observations in electrolysis—and his friend William Whewell helped with the words. Then later, Svante Arrhenius proposed that salts dissociate into ions in solution, earning a Nobel in 1903.
So yeah, ions: simple in concept, but born out of experiments, etymology, and Nobel-worthy insights.
Types of Ions: Monatomic, Polyatomic, and Even Zwitterions
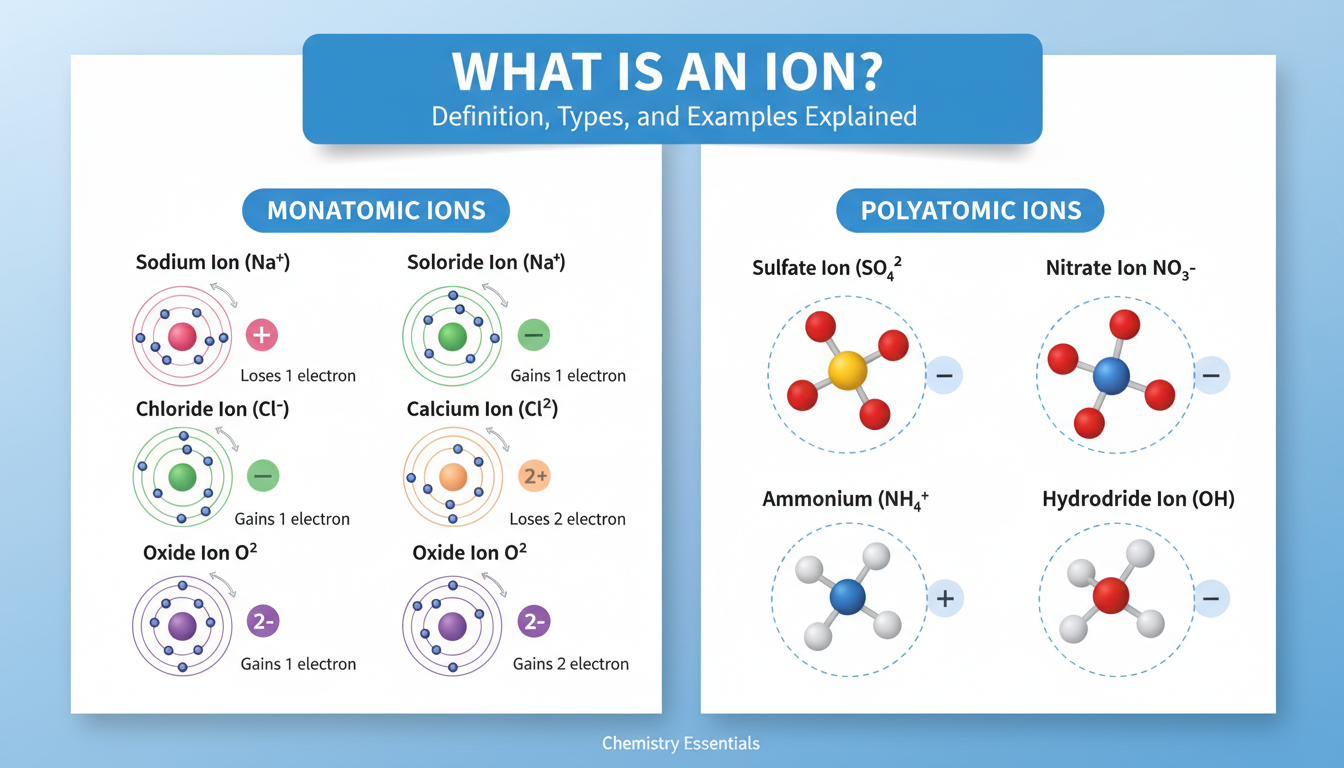
Monatomic Ions
These are your simplest kind—one atom with a charge. Think or , and an entire periodic table’s worth of similar examples. Cations like magnesium or calcium, anions like oxide or sulfide—these are staples in chemistry.
Polyatomic Ions
Then there are ions built from multiple atoms. A classic is ammonium (), or sulfate (). These usually behave as single charged units, and show up in household chemicals and biological discussions.
Zwitterions
A slight twist: zwitterions carry both charges in one molecule—neutral overall, but internally charged. Amino acids at certain pH levels? Yup, those are zwitterions.
Why Ions Matter: Real-World Examples
Everyday Life—Water, Salt, and Batteries
Remember dissolving table salt in water in grade school? That dissociation forms and , which conduct electricity—that’s why salty water works in circuits (or corpses, in crazy sci-fi scenarios). Ions underlie the functioning of batteries too—like lithium-ion ones powering your laptop and phone.
Biological Importance—Electrolytes at Work
Ions like sodium, potassium, and calcium are essential electrolytes in our bodies. They orchestrate nerve impulses, muscle contraction, and fluid balance. Without them, nerves misfire, muscles don’t move—you get the picture.
Industrial and Technological Uses
- Air purifiers often use ions to disrupt harmful microbes.
- Mass spectrometry and particle accelerators rely on controlled ion streams.
- Ionosphere phenomena, auroras—those mesmerizing space lights—are thanks to ions up high in the atmosphere.
“Ions are the unsung carriers—in solutions, cell signals, and even in the glow of a neon lamp.”
(Imagine a chemist saying that in a slightly distracted tone while stirring coffee—because ions, they’re everywhere.)
How Ions Form: Ionization Explained
By Losing or Gaining Electrons
The most usual route to an ion is losing or gaining electrons. Metals tend to lose electrons and become positive ions; nonmetals often gain electrons to become negative ions. That’s how salts form—metal meets nonmetal, electrons shift, boom—ionic bond.
Through Radiation or Collisions
Ions can also form when atoms get hit by a high-energy particle or radiation (like UV or X-rays). That’s called ionization, and it’s why the air conducts electricity in space, and why radiation detectors work.
Ionization Energy Variation
Different atoms require different energies to ionize. Sodium has low ionization energy—easily loses an electron; helium, high energy—harder to ionize. This influences chemistry and reactivity in big ways.
Quick Summary of Ion Types and Formation
- Cations: lost electrons, positive charge (e.g., )
- Anions: gained electrons, negative charge (e.g., )
- Monatomic vs. polyatomic vs. zwitterion structures
- Formation via electron transfer or energetic interactions
Conclusion
Ions are deceptively simple and yet foundational. From the table salt that seasons our meals to the neurochemical dance in our brains, they’re everywhere. They give salts taste, enable batteries, stabilize water, run nerves—and even make a neon sign glow. Appreciating ions is to glimpse the charged strangeness of the universe itself.
FAQs
What exactly makes an ion different from a neutral atom?
An ion has unequal numbers of protons and electrons, giving it a net electric charge; a neutral atom has them balanced. Even losing or gaining a single electron creates an ion.
Why are cations and anions important in everyday life?
They conduct electricity in solutions, help muscles and nerves function, enable batteries, and form common compounds like table salt—a major part of daily existence.
Can one molecule be both positive and negative at the same time?
Yes—that’s a zwitterion. It has separate positively and negatively charged regions but is overall neutral. Amino acids at certain pH levels are common examples.
How do ions form in radiation?
Radiation or high-energy collisions can knock electrons off atoms, forming ions—a process used in detectors and seen in phenomena like the aurora.
Are all ions harmful or reactive?
Not necessarily. Some ions, like electrolytes in physiology, are essential; others, like radicals, are highly reactive. Context matters—ions are tools, not villains.
Do ions only exist in water?
Not at all. They thrive in solution but can also exist in gases, plasmas, and even solids within ionic lattices, like crystalline salts.
This exploration shows that ions, simple though they seem, are messengers, connectors, and agents of change—from the microscopic drama in cells to cosmic light shows above. They’re quietly indispensable.


 February 8, 2026
February 8, 2026  5 Min
5 Min  No Comment
No Comment