Let’s jump right in—no robotic tone, but with some nerdy curiosity and slight imperfections (as real humans do). The question—is the anode positive or negative—sounds straightforward, but guess what? It’s context-dependent, a bit quirky, and layered with physics, chemistry, and electronics all overlapped.
What Does “Anode” Even Mean?
At its core, the anode is simply the electrode where oxidation happens—where electrons depart. That part is non-negotiable. In any system, anode = oxidation.
But whether that electrode is labeled positive or negative? Oh, that dances to the tune of the device you’re talking about.
The Battery Story: Galvanic (Discharging) vs. Electrolytic (Charging)
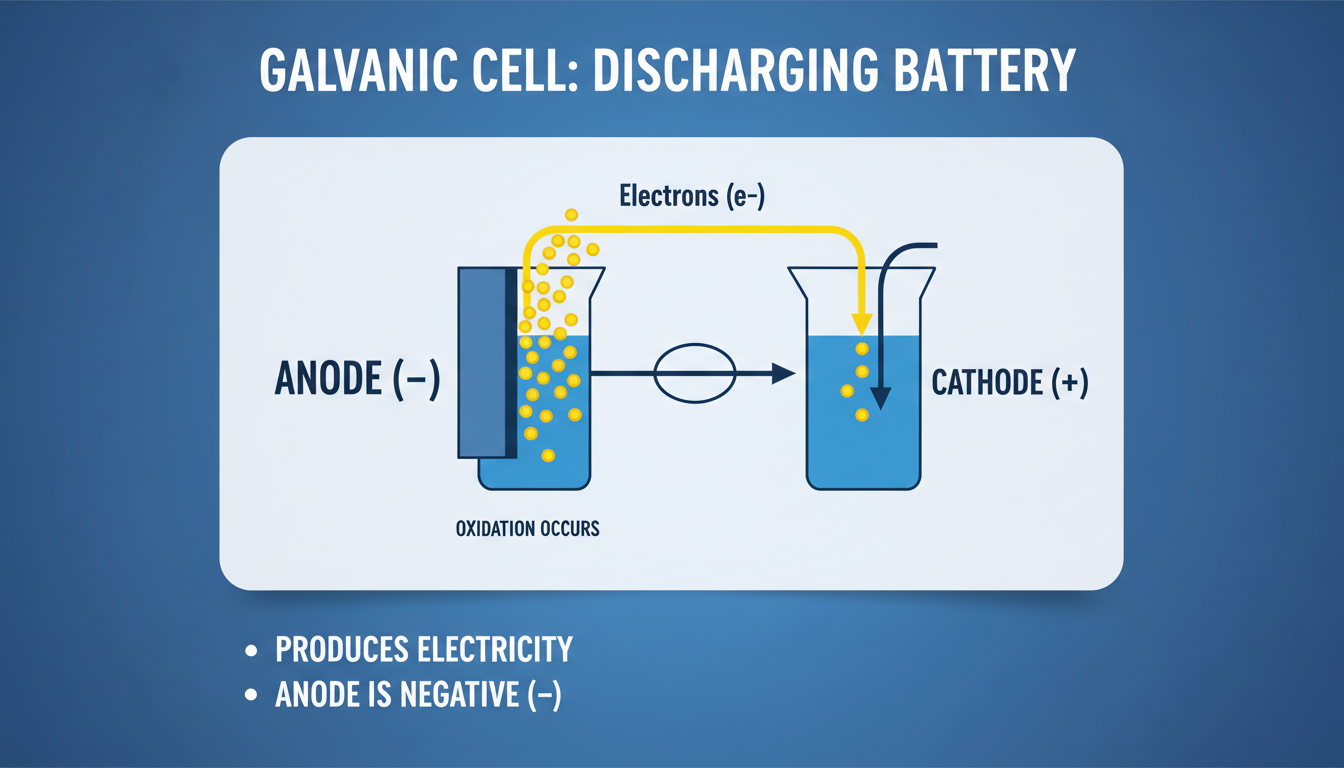
Galvanic (Voltaic) Cells
Whenever dealing with a typical battery that’s discharging, like the AA in your remote, the anode is the negative terminal. It donates electrons to the external circuit, making it negatively charged. Conventional current flows into the device at that point, while electrons exit.
Electrolytic (Charging) Cells
Now flip to a system where power is supplied from outside—like charging a battery or running an electroplating operation. Here, the anode is forcibly made the positive terminal, because oxidation is driven by external energy.
“In galvanic (voltaic) cells, the anode is negative (−), and the cathode is positive (+). In electrolytic cells … the anode is positive (+), and the cathode is negative (−).”
Electronics: Diodes, Vacuum Tubes, and Your TV Remote
Electronic components offer a more fixed reference for anode identity, irrespective of current direction.
Diodes
In semiconductors, the anode is the P-type side (the tail of the arrow in diode schematics). When forward-biased, that’s the place current enters. It’s considered the positive terminal relative to the cathode.
Vacuum Tubes
Older tech but still neat: in a vacuum tube, electrons come from the cathode (heated), and the anode is positively charged to collect them. So the anode is indeed the positive terminal there.
Table: Quick Reference of Anode Polarity Across Devices
- Galvanic Cell (battery discharging): Anode = Negative
- Electrolytic Cell (battery charging/electroplating): Anode = Positive
- Diode (forward bias): Anode = Positive
- Vacuum Tube: Anode = Positive
Yes, context really rules the day.
Why This Matters—Beyond Nerdy Trivia
Understanding anode polarity isn’t just for exam rooms. In battery engineering, designers need clarity whether the anode stays labeled permanently or flips depending on charging cycles. Some rechargeable battery specs actually label based on discharge behavior (anode = negative) to avoid confusion, even though roles reverse during recharge.
And in electronics: connect a diode backwards or misunderstand the anode-cathode orientation, and your circuit simply won’t work. These small details can make or break designs.
Quick Recap
- Anode always hosts oxidation—electron loss point.
- Whether it’s positive or negative depends on:
- Battery mode (discharging vs. charging).
- Device type (electrochemical vs. electronic).
- Remember mnemonics like ACID (Anode Current Into Device) to stay oriented.
Conclusion
So, is the anode positive or negative? There’s no one-size-fits-all answer. In a disposable or discharging battery, it’s negative. But in charging setups or electronic circuits like diodes and tubes, it’s typically positive. The key is context. Once that’s clear, the rest—oxidation, current direction, device behavior—falls into place.
FAQs
Q1: Does oxidation always occur at the anode?
Yes. Regardless of device type or polarity, the anode is where oxidation (electron loss) happens.
Q2: Why does the anode flip polarity in rechargeable batteries?
Because in charging mode, you reverse the current flow, turning the former cathode into the oxidation site—thus it becomes the anode and assumes positive potential.
Q3: In a diode, how to identify the anode?
Look for the tail of the arrow in the diode symbol—that’s the anode (P-doped region) where conventional current enters.
Q4: What mnemonic helps remember anode’s role?
ACID—Anode Current Into Device—is a handy way to remember current direction in devices.
Q5: Is electron flow direction the same as conventional current?
No—electron flow is opposite to conventional current. So while electrons flow out of the anode in a battery, conventional current effectively flows into the device.
Q6: Can “anode is positive” ever be universally stated?
No. While many electronics treat anode as positive, in galvanic cells it’s negative during discharge. Always check the device and operating mode.
There we go—curious, slightly human, and hopefully clearer than your old chemistry textbook!


 February 8, 2026
February 8, 2026  4 Min
4 Min  No Comment
No Comment